Animal groups smell a rat on government health food claims

On July 27, EAST and legislator Lin Shu-Fen held a joint press conference at the Legislative Yuan to condemn a Ministry of Health and Welfare (MOHW) plan to expand the scope of animal testing for health food claims.
Taiwan’s health food certification label—marked by a depiction of an exuberant green man—has long violated international norms by endorsing health food claims proven only in animal trials.
On May 25, the MOHW’s Food and Drug Administration (TFDA) unveiled plans to incorporate osteoarthritis-related health claims into the scheme, adding three new animal testing items to those already on the books. The proposed animal tests involve cruel and unnecessary procedures on rats including removal of anterior cruciate ligaments, excision of medial menisci, and injection of joint deterioration-causing chemicals.
While international norms require that purported health effects are confirmed in human trials to ensure they apply to human consumers, countless products have made it to market in Taiwan carrying claims based solely on animal experiments. There are currently 13 health claims that can be certified under Taiwan’s health food certification scheme, with the majority able to be certified using only animal experiments.
Internationally, the relevant European and U.S. codes and the widely-acknowledged Codex Alimentarius (‘Food Code’) all insist that health claims must be based on evidence from human trials, and will not accept animal testing data as independent evidence.
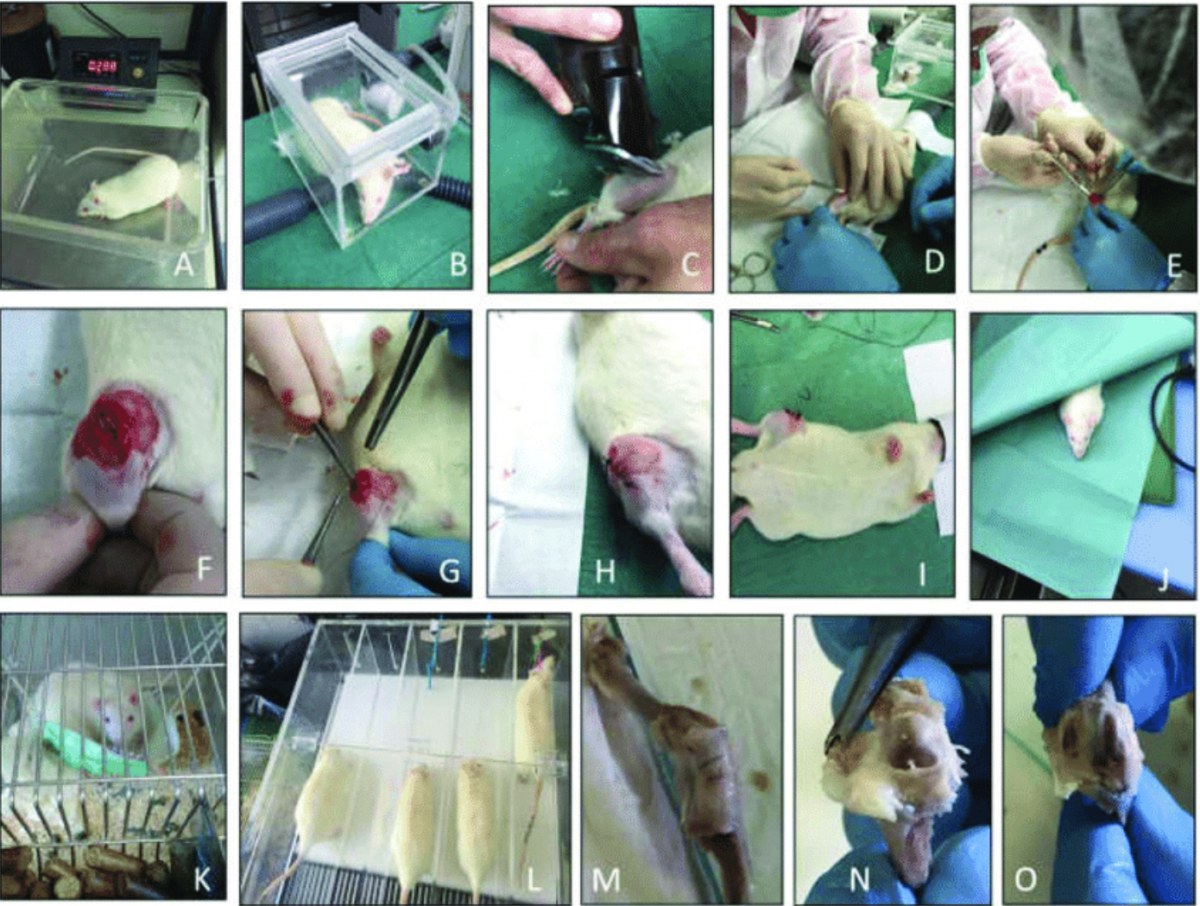
Source: International Journal of Molecular Sciences 2019
“Rats are unable to effectively simulate human osteoarthritis,” noted EAST researcher Hsin-Yi (Sally) Yao.
“Osteoarthritis scarcely occurs naturally in rats, and drugs that effectively treat arthritis in rats have repeatedly failed in human clinical trials. What’s more, the draft released by the MOHW requires the use of ten-week old rats whose bones have only just fully developed, while osteoarthritis usually occurs after middle age in human patients.”
The MOHW’s flawed study design also requires the animal subjects be fed health foods just one day after they have been induced with arthritis. Animal models compiled by EAST show that osteoarthritis symptoms take up to 10 weeks to develop in rats after they are given the disease by injection or surgical operation, with the MOHW’s own draft noting that early signs can take two to six weeks to appear.
Further, while osteoarthritis in humans disproportionately affects women, the proposed study design requires the use of only male rats, ignoring the effects of one of the most important risk factors.
A host of reliable and accessible human tests are already available, including measuring incapacitance with force plates and gait analyzers, utilizing MRI technology to observe articular cartilage lesions, and monitoring knee width changes with simple physical exams in routine follow-up appointments. Additionally, recent years have seen the development of innovative non-animal methods that reproduce the natural development of osteoarthritis in humans, such as cartilage-on-a-chip and osteochondral plug models.
Legislator Lin Shu-Fen expressed consternation at the MOHW’s plan, saying “As the world moves rapidly to require human trials for claims made about health food products, I fail to see why Taiwan seems intent on remaining behind the curve.”
“If the MOHW wishes to promote the competitiveness of Taiwan’s biomedical research and development sectors, it should actively encourage the adoption of emerging human cell models” added Ms. Yao.
EAST and legislator Lin Shu-Fen call on the MOHW to urgently revise the proposed draft and the methods used to assess the 13 health claims covered by Taiwan’s health food certification scheme, replacing unnecessary animal tests with human or non-animal testing methods.
Additionally, we urge the Council of Agriculture to ensure that non-animal methods are used whenever available in line with Taiwan’s animal protection laws, and strengthen oversight of research institutions.
Media contact
Hsin-Yi (Sally) Yao, Researcher
Environment & Animal Society of Taiwan (EAST)
Mobile: +886 (9) 3559 6996
Phone: +886 (2) 2236 9735










